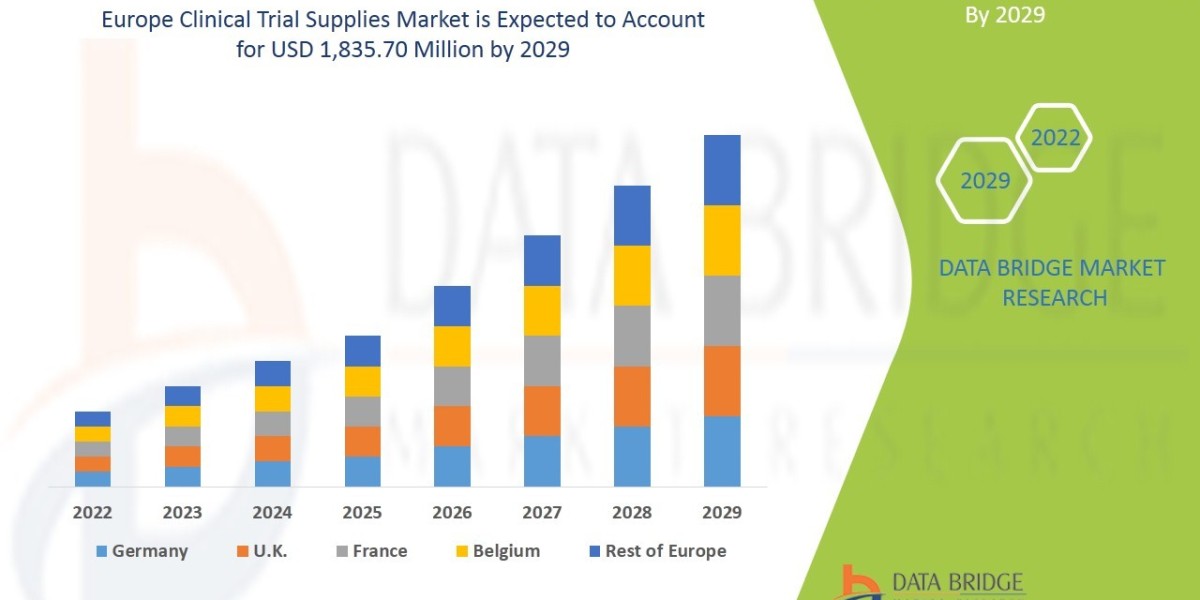
Executive Summary Europe Clinical Trial Supplies Market
Data Bridge Market Research analyses that the market is growing with a CAGR of 7.8% in the forecast period of 2022 to 2029 and is expected to reach USD 1,835.70 million by 2029.
Europe Clinical Trial Supplies Market report provides the market potential for each geographical region based on the growth rate, macroeconomic parameters, consumer buying patterns, and market demand and supply scenarios. The report focuses on the top players in North America, Europe, Asia-Pacific, South America, and Middle East & Africa. Europe Clinical Trial Supplies Market document delivers an extensive research on the current conditions of the industry, potential of the market in the present and the future prospects from various points of views. The numerical and statistical data has been denoted in the graphical format for a clear understanding of facts and figures.
The analysis covered in the global Europe Clinical Trial Supplies Market report gives an assessment of various segments that are relied upon to witness the quickest development amid the approximated forecast frame. The market study encompasses a market attractiveness analysis, wherein each segment is benchmarked based on its market size, growth rate, and general attractiveness. All the information, facts, and statistics covered in the report lead to actionable ideas, improved decision-making and better deciding business strategies. Europe Clinical Trial Supplies Market report contains historic data, present market trends, environment, technological innovation, upcoming technologies and the technical progress in the related industry.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Europe Clinical Trial Supplies Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/europe-clinical-trial-supplies-market
Europe Clinical Trial Supplies Market Overview
**Segments**
- On the basis of product type, the Europe Clinical Trial Supplies Market can be segmented into manufacturing, packaging, and labeling. The manufacturing segment includes services related to drug formulation, labeling, and packaging. The packaging segment consists of primary and secondary packaging solutions for clinical trial supplies, ensuring proper storage and transportation. The labeling segment involves the design and printing of labels that comply with regulatory requirements and provide essential information about the trial supplies.
- With regards to application, the market can be segmented into oncology, cardiovascular diseases, neurology, and others. Oncology trials dominate the market due to the high prevalence of cancer in Europe and the increasing number of clinical trials focused on cancer treatments. The cardiovascular diseases segment is also significant, driven by the rising incidence of heart diseases in the region. Neurology trials are gaining traction, particularly in the research of conditions like Alzheimer's and Parkinson's disease.
- Based on end-user, the Europe Clinical Trial Supplies Market can be categorized into pharmaceutical and biotechnology companies, contract research organizations (CROs), and research institutes. Pharmaceutical and biotechnology companies are the primary end-users, investing heavily in clinical trials to bring new drugs to market. CROs play a crucial role by providing specialized services for clinical trial supplies management. Research institutes also contribute to the market by conducting academic and government-funded trials.
**Market Players**
- Some key players in the Europe Clinical Trial Supplies Market include Catalent, Inc., PCI Pharma Services, Almac Group, Thermo Fisher Scientific Inc., Sharp, Biocair, PAREXEL International Corporation, KLIFO A/S, Movianto, and Ancillare, LP. These companies offer a wide range of services related to clinical trial supplies, from manufacturing and packaging to logistics and distribution. They have established a strong presence in Europe through strategic partnerships and collaborations with pharmaceutical companies and research organizations.
- Another group of market players comprises smaller companies and startups that specialize in niche areas of clinical trial supplies, such as temperature-controlled packaging, direct-to-patient distribution, and virtual trials support. These players bring innovation and agility to the market, catering to the evolving needs of clinical trial sponsors and investigators. Overall, the Europe Clinical Trial Supplies Market is characterized by a mix of large multinational companies and smaller, more specialized providers, creating a competitive landscape that drives innovation and quality in clinical trial services.
The Europe Clinical Trial Supplies Market is experiencing significant growth driven by various factors such as increasing research and development activities in the pharmaceutical and biotechnology sectors, rising prevalence of chronic diseases, and the focus on personalized medicine. In addition to the traditional segments of manufacturing, packaging, and labeling, new trends are emerging in the market that are shaping its landscape. One notable trend is the growing demand for direct-to-patient distribution services, driven by the need for patient-centric approaches in clinical trials. This trend allows for more convenient and efficient delivery of trial supplies to participants, enhancing patient recruitment and retention rates.
Furthermore, the market is witnessing a shift towards the adoption of innovative technologies such as blockchain and artificial intelligence to optimize supply chain management and ensure regulatory compliance. These technologies offer enhanced traceability, transparency, and security in the distribution of clinical trial supplies, addressing key challenges faced by stakeholders in the industry. Moreover, the increasing focus on sustainability and environmental responsibility is driving the implementation of eco-friendly packaging solutions in clinical trials. Companies are exploring alternative materials and green packaging practices to reduce their carbon footprint and contribute to environmental conservation efforts.
Another significant aspect shaping the Europe Clinical Trial Supplies Market is the regulatory landscape governing clinical trials. The European Medicines Agency (EMA) and other regulatory bodies have stringent requirements for the distribution and handling of trial supplies to ensure patient safety and data integrity. Compliance with these regulations is crucial for market players to avoid penalties and maintain their reputation in the industry. As a result, there is a growing emphasis on quality assurance and regulatory compliance in the provision of clinical trial supply services, with companies investing in training and infrastructure to meet the evolving regulatory standards.
In conclusion, the Europe Clinical Trial Supplies Market is dynamic and evolving, driven by advancements in technology, changing patient preferences, and regulatory requirements. Market players need to remain agile and adaptable to capitalize on emerging opportunities and address potential challenges in the industry. By staying abreast of market trends, embracing innovation, and prioritizing quality and compliance, companies can position themselves for sustained growth and success in the competitive landscape of the Europe Clinical Trial Supplies Market.The Europe Clinical Trial Supplies Market is witnessing significant growth propelled by various factors such as increased research and development activities in the pharmaceutical and biotechnology sectors, as well as the rising prevalence of chronic diseases. The focus on personalized medicine is also contributing to the expansion of the market. In addition to traditional segments like manufacturing, packaging, and labeling, new trends are emerging that are reshaping the industry landscape. One notable trend is the rising demand for direct-to-patient distribution services, aiming at enhancing patient recruitment and retention rates by providing more convenient and efficient delivery of trial supplies to participants.
Moreover, there is a notable shift towards the adoption of innovative technologies such as blockchain and artificial intelligence to optimize supply chain management and ensure regulatory compliance in the Europe Clinical Trial Supplies Market. These technologies offer improved traceability, transparency, and security in the distribution of clinical trial supplies, addressing critical challenges faced by stakeholders in the industry. Additionally, the industry is increasingly focusing on sustainability and environmental responsibility, leading to the implementation of eco-friendly packaging solutions in clinical trials. Companies are exploring alternative materials and green packaging practices to reduce their carbon footprint and contribute to environmental conservation efforts.
The regulatory landscape governing clinical trials, overseen by entities like the European Medicines Agency (EMA) and other regulatory bodies, plays a pivotal role in shaping the Europe Clinical Trial Supplies Market. These bodies have stringent requirements for the distribution and handling of trial supplies to ensure patient safety and data integrity. Compliance with these regulations is paramount for market players to avoid penalties and safeguard their reputation within the industry. Consequently, there is a growing emphasis on quality assurance and regulatory compliance in the provision of clinical trial supply services, with companies investing in training and infrastructure to meet the evolving regulatory standards.
In summary, the Europe Clinical Trial Supplies Market is dynamic and evolving, driven by technological advancements, changing patient preferences, and regulatory requirements. Market players must demonstrate agility and adaptability to capitalize on emerging opportunities and tackle potential challenges in the industry. Keeping abreast of market trends, fostering innovation, and prioritizing quality and compliance are essential strategies for companies looking to achieve sustained growth and success in the competitive landscape of the Europe Clinical Trial Supplies Market.
The Europe Clinical Trial Supplies Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/europe-clinical-trial-supplies-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
What insights readers can gather from the Europe Clinical Trial Supplies Market report?
- Learn the behavior pattern of every Europe Clinical Trial Supplies Market -product launches, expansions, collaborations and acquisitions in the market currently.
- Examine and study the progress outlook of the global Europe Clinical Trial Supplies Market landscape, which includes, revenue, production & consumption and historical & forecast.
- Understand important drivers, restraints, opportunities and trends (DROT Analysis).
- Important trends, such as carbon footprint, R&D developments, prototype technologies, and globalization.
Browse More Reports:
Global Offshore Mooring Systems Market
Global Industrial Backhoe Loader Market
Global Hybrid and Community Cloud as a Service Market
North America Stand-Up Paddleboard Market
Global Eggshell Membrane Powder Market
Global Aluminium Composite Panels Market
Global Bulk Chemical Drums Market
Asia-Pacific Pharmaceuticals Packaging Testing Equipment Market
Global Composite Materials Market
Global Semi-Finished Pastry Ingredients Market
Global Fragrance Packaging Market
Global Electrical Discharge Machine (EDM) Market
Global Bio-Based Polypropylene (PP) Market
Global Flat Back Tape Market
Global 3D Laser Scanner Market
North America Freight Transportation Management Market
Asia-Pacific API Intermediates Market
North America Defibrillators Market
Asia-Pacific Smoked Cheese Market
Europe Soft Tissue Repair Market
Global Crambe Abyssinica Seed Oil Market
Europe Anthrax Treatment Market
Global Dog Food Extrusion Market
Global Waterproof Coatings and Membranes Market
Australia and New Zealand Non-Stick Cookware Market
Global Cerebral Palsy Market
Global Snow Pushers Market
Global Electro Pneumatic Train Brakes Market
Global Loyalty Management Market
Global Connected Living Room Market
Global Specialty Oilfield Chemicals Market
Global Botanical Ingredients for Neutraceutical Market
Global Residential Portable Air Purifier Market
North America Chinese Hamster Ovary (CHO) Cells Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
Europe Clinical Trial Supplies Market, Europe Clinical Trial Supplies Market Trends, Europe Clinical Trial Supplies Market Growth, Europe Clinical Trial Supplies Market Demand, Europe Clinical Trial Supplies Market Size, Europe Clinical Trial Supplies Market Scope, Europe Clinical Trial Supplies Market Insights, Europe Clinical Trial Supplies Market Analysis