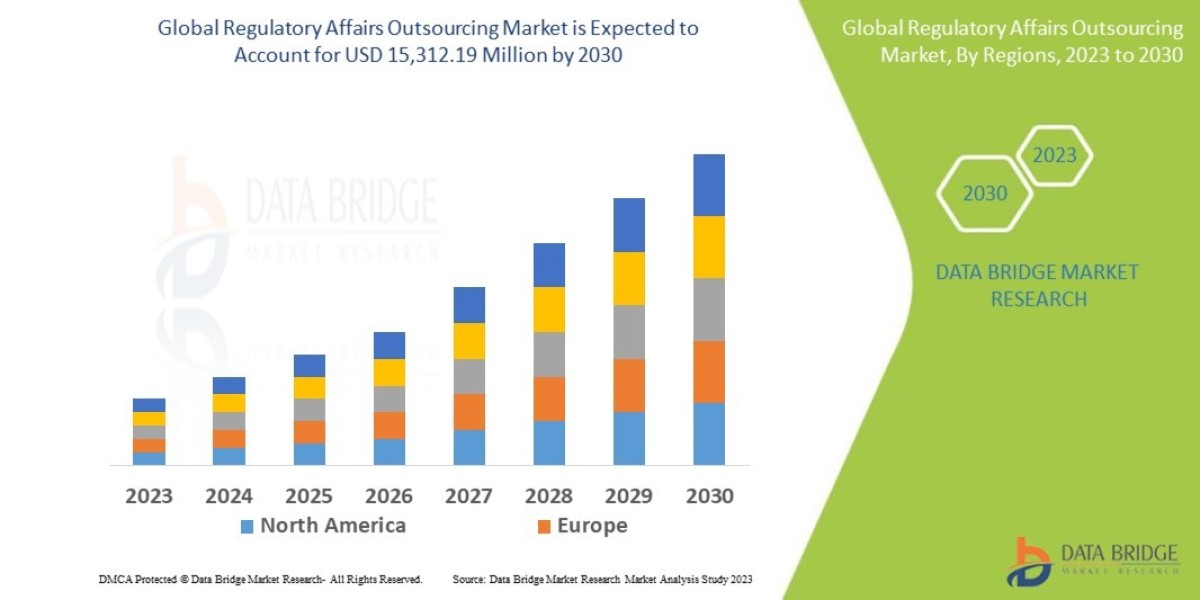
"Executive Summary Regulatory Affairs Outsourcing Market : Data Bridge Market Research analyses that the regulatory affairs outsourcing market which was USD 9,389.20 million in 2022, would rocket up to USD 15,312.19 million by 2030, and is expected to undergo a CAGR of 10.66% during the forecast period.
The Regulatory Affairs Outsourcing Market report has been designed in such a way that it proves to be the most appropriate to the business needs. Moreover, this market report gives idea to clients about the market drivers and restraints with the help of SWOT analysis and also provides all the CAGR projections for the historic year, base year and forecast period. This Regulatory Affairs Outsourcing Market study also evaluates the market status, market share, growth rate, future trends, market drivers, opportunities and challenges, risks and entry barriers, sales channels, distributors and Porter's Five Forces Analysis.
The Regulatory Affairs Outsourcing Market business report endows with an exhaustive overview of product specification, technology, product type and production analysis considering major factors such as revenue, costing, and gross margin. This market report also provides the list of leading competitors along with the strategic insights and analysis of the key factors influencing the industry. Regulatory Affairs Outsourcing Market research study lends a hand to the purchaser in comprehending the various drivers and restraints with their effects on the market during the forecast period. The Regulatory Affairs Outsourcing Market industry report comprises of primary, secondary and advanced information about the global market with respect to status, trends, size, share, growth, and segments in the forecasted
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Regulatory Affairs Outsourcing Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/global-regulatory-affairs-outsourcing-market
Regulatory Affairs Outsourcing Market Overview
**Segments**
- Based on services, the regulatory affairs outsourcing market can be segmented into regulatory writing and publishing, clinical trial applications, regulatory consulting and legal representations, product registration and clinical trial applications, regulatory submissions, and others. The regulatory writing and publishing segment is expected to witness significant growth due to the increasing demand for accurate regulatory documentation in the healthcare industry. Regulatory consulting and legal representations are also crucial services, ensuring compliance with various regulations and standards across different regions. Product registration and clinical trial applications are essential for launching new products in the market, driving the growth of this segment.
- On the basis of application, the regulatory affairs outsourcing market is categorized into pharmaceutical, biotechnology, and medical devices. The pharmaceutical sector dominates the market due to the complex regulatory processes involved in drug development and approval. Regulatory affairs outsourcing helps pharmaceutical companies streamline their regulatory activities and ensure compliance with global regulations. The biotechnology industry is also a key segment, as these companies often require specialized regulatory expertise to navigate the regulatory landscape. The medical devices sector is witnessing rapid growth, creating opportunities for regulatory affairs outsourcing companies to support manufacturers in obtaining regulatory approvals for their products.
- By end-user, the regulatory affairs outsourcing market is segmented into contract research organizations (CROs), pharmaceutical companies, biotechnology companies, and medical device companies. CROs play a vital role in conducting clinical trials and other research activities on behalf of pharmaceutical, biotechnology, and medical device companies. These organizations often outsource regulatory affairs services to specialized providers to ensure compliance and efficiency in their operations. Pharmaceutical companies, biotechnology companies, and medical device companies rely on regulatory affairs outsourcing to navigate the complex regulatory requirements across different markets and expedite product approvals.
**Market Players**
- Some of the key players operating in the global regulatory affairs outsourcing market include:
- ICON plc
- PAREXEL International Corporation
- PRA Health Sciences
- Covance Inc.
- Freyr Solutions
- Wuxi AppTec
- Criterium, Inc.
- Accell Clinical Research
- ProductLife Group
- Cytel Inc.
These market players are focusing on strategic collaborations, acquisitions, and partnerships to enhance their service offerings and expand their geographic presence in the regulatory affairs outsourcing market. The increasing demand for regulatory expertise and the growing complexity of regulatory requirements are driving the expansion of these companies as they aim to fulfill the diverse needs of their clients worldwide.
In the global regulatory affairs outsourcing market, we can see a clear trend towards the growing importance of regulatory writing and publishing services. As the healthcare industry continues to evolve and face stringent regulatory requirements, the need for accurate and compliant documentation has become paramount. Companies are increasingly relying on outsourcing partners to handle regulatory writing and publishing tasks to ensure that their submissions meet all regulatory standards. This segment is expected to witness significant growth as more companies recognize the value of expertly crafted regulatory documents in gaining approvals and maintaining compliance.
Another key area of focus in the regulatory affairs outsourcing market is the application segment, particularly within the pharmaceutical sector. With the complex nature of drug development and approval processes, pharmaceutical companies are turning to outsourcing partners to navigate the intricate regulatory landscape. By outsourcing regulatory activities, these companies can streamline their operations and ensure timely approvals for their products. The biotechnology and medical devices sectors also present significant opportunities for regulatory affairs outsourcing, as specialized expertise is often required to meet the unique regulatory challenges faced by companies in these industries.
When considering the end-user segment of the regulatory affairs outsourcing market, it is evident that contract research organizations (CROs) play a crucial role in driving demand for outsourced regulatory services. CROs rely on outsourcing partners to ensure compliance in their clinical trial operations and research activities, allowing them to focus on their core competencies. Pharmaceutical, biotechnology, and medical device companies also heavily rely on regulatory affairs outsourcing to navigate the complexities of global regulatory requirements and accelerate product approvals. By partnering with specialized providers, these companies can leverage external expertise to efficiently manage their regulatory affairs activities.
In terms of market players, the global regulatory affairs outsourcing market is dominated by established companies such as ICON plc, PAREXEL International Corporation, and PRA Health Sciences. These key players have been actively pursuing strategic initiatives such as collaborations and acquisitions to strengthen their service offerings and expand their geographic reach. By staying at the forefront of regulatory trends and requirements, these market players are well-positioned to meet the evolving needs of their clients and drive growth in the regulatory affairs outsourcing market.
In conclusion, the regulatory affairs outsourcing market continues to witness substantial growth driven by the increasing demand for regulatory expertise across various industries. Companies are increasingly recognizing the value of outsourcing regulatory activities to specialized providers to ensure compliance, expedite approvals, and navigate the complex regulatory landscape efficiently. As market players continue to innovate and expand their service offerings, we can expect the regulatory affairs outsourcing market to further evolve to meet the dynamic needs of the healthcare industry and beyond.The regulatory affairs outsourcing market is witnessing notable growth and evolution, particularly driven by the increasing emphasis on regulatory writing and publishing services. With the healthcare industry facing stringent regulations, companies are recognizing the importance of accurate and compliant documentation for gaining approvals and maintaining compliance. Outsourcing partners play a crucial role in this space, offering expertise in crafting regulatory documents that meet global standards. The demand for regulatory writing and publishing services is expected to continue rising as companies strive to navigate complex regulatory landscapes effectively.
Furthermore, the pharmaceutical sector remains a dominant segment within the regulatory affairs outsourcing market. Due to the intricate nature of drug development and approval processes, pharmaceutical companies rely on outsourcing partners to streamline regulatory activities and ensure timely approvals. The biotechnology and medical devices industries also present significant opportunities for regulatory affairs outsourcing, as these sectors require specialized expertise to address their unique regulatory challenges. By collaborating with outsourcing providers, companies in these industries can leverage external knowledge to manage regulatory complexities efficiently.
In terms of end-users, contract research organizations (CROs) play a pivotal role in driving the demand for outsourced regulatory services. By outsourcing regulatory activities, CROs can ensure compliance in their clinical trial operations and research endeavors, allowing them to focus on core competencies. Pharmaceutical, biotechnology, and medical device companies also heavily rely on regulatory affairs outsourcing to navigate global regulations and accelerate product approvals. Specialized providers offer valuable support in managing regulatory requirements across different markets, enabling companies to expedite approvals and enhance operational efficiency.
Key market players such as ICON plc, PAREXEL International Corporation, and PRA Health Sciences are at the forefront of the regulatory affairs outsourcing market, actively engaging in strategic initiatives to enhance their service offerings and expand their geographical presence. Through collaborations and acquisitions, these established companies aim to strengthen their capabilities and cater to the evolving needs of clients in a rapidly changing regulatory landscape. By staying abreast of regulatory trends and requirements, market leaders are well-positioned to drive growth and innovation in the regulatory affairs outsourcing space.
In summary, the regulatory affairs outsourcing market is experiencing significant growth momentum fueled by the rising demand for regulatory expertise across diverse industries. As companies continue to recognize the benefits of outsourcing regulatory activities, the market is poised to evolve further to meet the dynamic needs of the healthcare sector and beyond. With a focus on regulatory writing, pharmaceutical applications, and diverse end-user segments, the regulatory affairs outsourcing market is set for continuous expansion and adaptation to drive compliance and efficiency in regulatory processes.
The Regulatory Affairs Outsourcing Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/global-regulatory-affairs-outsourcing-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
Key Influence of this Regulatory Affairs Outsourcing Market:
- Comprehensive assessment of all opportunities and risk in this Regulatory Affairs Outsourcing Market
- This Regulatory Affairs Outsourcing Marketrecent innovations and major events
- Detailed study of business strategies for growth of the this Regulatory Affairs Outsourcing Market leading players
- Conclusive study about the growth plot of the Market for forthcoming years
- In-depth understanding of this Regulatory Affairs Outsourcing Market particular drivers, constraints and major micro markets
- Favorable impression inside vital technological and market latest trends striking this Regulatory Affairs Outsourcing Market
- To provide historical and forecast revenue of the Regulatory Affairs Outsourcing Marketsegments and sub-segments with respect to four main geographies and their countries- North America, Europe, Asia, and Rest of the World (ROW)
- To provide country level analysis of the Regulatory Affairs Outsourcing Market t with respect to the current market size and future prospective
Browse More Reports:
North America Personal Care Ingredients Market
Global FinFET Technology Market
Global Paper Dyes Market
Asia-Pacific Protein Hydrolysates Market
Global Inline Metrology Market
North America Retail Analytics Market
Global Thrombosis Drug Market
Europe Network Test Lab Automation Market
Global Perinatal Infections Market
Global Light-Emitting Diode (LED) Probing and Testing Equipment Market
Global Mobile Campaign Management Platform Market
Global Fruits and Vegetables Processing Equipment Market
Global STD Diagnostics Market
Asia-Pacific Microgrid Market
Global Fluoxetine Market
Global Food Drink Packaging Market
Global Electric Enclosure Market
Asia-Pacific Artificial Turf Market
Global Hand Wash Station Market
Global Prostate Cancer Antigen 3 (PCA3) Test Market
Asia-Pacific Hydrographic Survey Equipment Market
Global Cable Testing and Certification Market
Global Leather Handbags Market
Global Post-Bariatric Hypoglycemia Treatment Market
Europe pH Sensors Market
Global Linear Low-Density Polyethylene Market
Global Ketogenic Diet Food Market
Asia-Pacific Small Molecule Sterile Injectable Drugs Market
Global Prescriptive Analytics Market
Global Viral Transport Media Market
Middle East and Africa Composite Bearings Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
Tag
"